Background:
Myeloma patient outcomes have continuously improved with the introduction of proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), monoclonal antibodies (mAbs) recognizing cell surface proteins and, most recently, B-cell maturation antigen (BCMA)-targeted bispecifics and chimeric antigen receptor (CAR)-guided T-cells. However, the prognosis in triple-class refractory (TCR) disease that is refractory to PIs, IMiDs, and CD38 mAbs remains poor and, since BCMA-targeted CAR-Ts and bispecifics are not curative, quadruple-class refractory (QCR) disease that progresses during or after these therapies is an emerging challenge, indicating a persistent need for novel agents. Rho-associated coiled-coil containing protein kinases (ROCK1 and ROCK2) may represent novel potential targets since ROCK1 has been associated with a plasma cell leukemia-like transcriptional profile and as a myeloma dependency. Moreover, ROCK2 mediates expression of Interferon regulatory factor 4 (IRF4) and c-MYC, as well as Th17 responses that may contribute to an immune suppressive microenvironment.
Methods:
To examine the central hypothesis that targeting ROCK1/2 could be a novel and effective approach to myeloma therapy, we performed pre-clinical studies with the ROCK1/2 inhibitor belumosudil mesylate using drug-naïve and drug-resistant myeloma cell lines in vitro. Also, in vivo studies with immune correlates were performed in the Vk*MYC immune-competent syngeneic myeloma model, which has been reported to have a 67% positive predictive value for clinical efficacy in humans.
Results:
Belumosudil mesylate as a single agent reduced the viability of a panel of myeloma cell lines representing different molecular subtypes in a time- and concentration-dependent manner, with median inhibitory concentrations from 618 to 1,114 nM at 72 hours. These represent clinically relevant concentrations as the mean steady state C max for belumosudil mesylate given with food in studies of chronic graft-versus-host disease patients was 4.36 mM. Staining of myeloma cells with Annexin V and propidium iodide (PI) demonstrated that belumosudil induced appearance first of Annexin +/PI - (early apoptotic) cells, followed later by Annexin +/PI + (late apoptotic) and Annexin -/PI + (necrotic) cells, in a concentration-dependent manner. Studies of myeloma resistance mechanisms showed belumosudil largely overcame adhesion-mediated drug resistance, and was active against MM.1S and RPMI 8226 cells that are considered daratumumab-resistant. Moreover, belumosudil showed equal or even, in some cases, higher potency against bortezomib-, carfilzomib-, dexamethasone-, iberdomide-, lenalidomide-, melphalan-, and mezigdomide-resistant cell lines compared to their drug-naïve counterparts. Belumosudil induced in vitro killing of myeloma cells in a time- and dose-dependent manner independent of the presence of natural killer (NK) cells. Notably, when used in combination with the CD38 mAb isatuximab and in the presence of NK cells, belumosudil enhanced myeloma cell killing and prevented isatuximab-induced loss of CD38 expression.
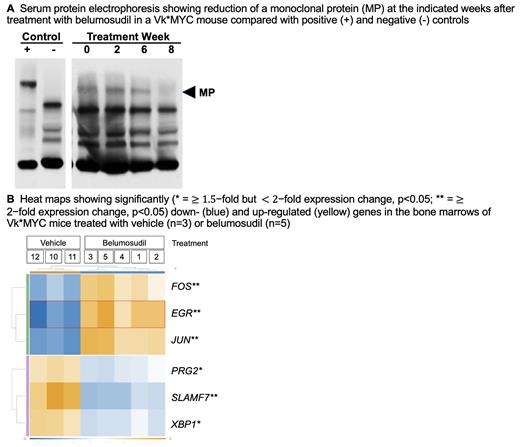
Mechanistically, belumosudil induced a reduction in c-MYC, IRF4, and phospho-STAT3 levels, consistent with its action on the IRF4/c-MYC axis. Dosing of belumosudil in vivo at 50 mg/kg twice weekly for three weeks in Vk*MYC mice with paraprotein levels consistent with active myeloma produced a consistent decline in disease burden as determined by serum protein electrophoresis monitoring ( A) compared to vehicle controls. NanoString analysis of tumor samples from these mice ( B) found down-regulation of XBP1 (X-box binding protein 1) and SLAMF7 (Signaling lymphocytic activation molecule family member 7), consistent with the anti-tumor efficacy of belumosudil mesylate. In addition, up-regulation was seen of c- JUN and c- FOS, consistent with promotion of T-cell activation and clonal expansion, as well as effector differentiation of antigen-activated CD8 + T cells.
Conclusions:
These pre-clinical in vitro and in vivo data support the hypothesis that targeting of ROCK1 and ROCK2 with belumosudil mesylate may be a promising strategy for relapsed/refractory myeloma and provide a rationale for its translation to the clinic. Currently, clinical trials of belumosudil are planned.
Disclosures
Meloni:Sanofi R&D: Current Employment, Current equity holder in publicly-traded company. Bouaboula:Sanofi R&D: Current Employment, Current equity holder in publicly-traded company. Bisht:Sanofi R&D: Current Employment, Current equity holder in publicly-traded company. Van de Velde:Sanofi: Current Employment, Current equity holder in publicly-traded company. Chiron:Sanofi R&D: Current Employment, Current equity holder in publicly-traded company. Virone-Oddos:Sanofi R&D: Current Employment, Current equity holder in publicly-traded company. Orlowski:Asylia Therapeutics: Current equity holder in private company, Patents & Royalties; Asylia Therapeutics, BioTheryX Inc., Heidelberg Pharma: Other: Laboratory Research Funding, Research Funding; BMS/Celgene Corporation, CARsgen Therapeutics, Exelixis Inc., Heidelberg Pharma, Janssen Biotech Inc., Sanofi/Genzyme, Takeda Pharmaceuticals USA Inc.: Other: Clinical Research Funding, Research Funding; AbbVie, Adaptive Biotech, Asylia Therapeutics, Inc., BioTheryX, Bristol-Myers Squibb Pharmaceuticals, Karyopharm Therapeutics, Meridian Therapeutics, Monte Rosa Therapeutics, Nanjing IASO Biotherapeutics, Neoleukin Corporation, Oncopeptides AB, Pfizer, In: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal